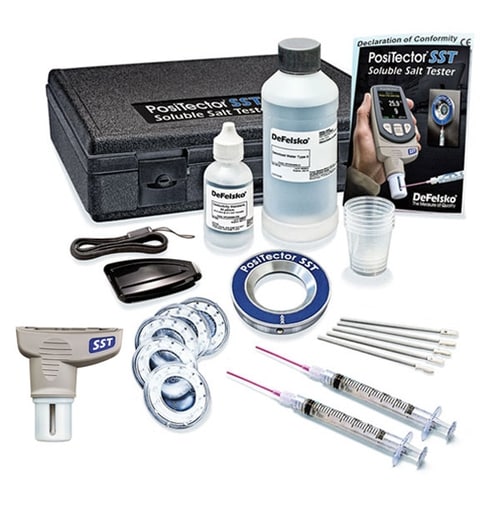
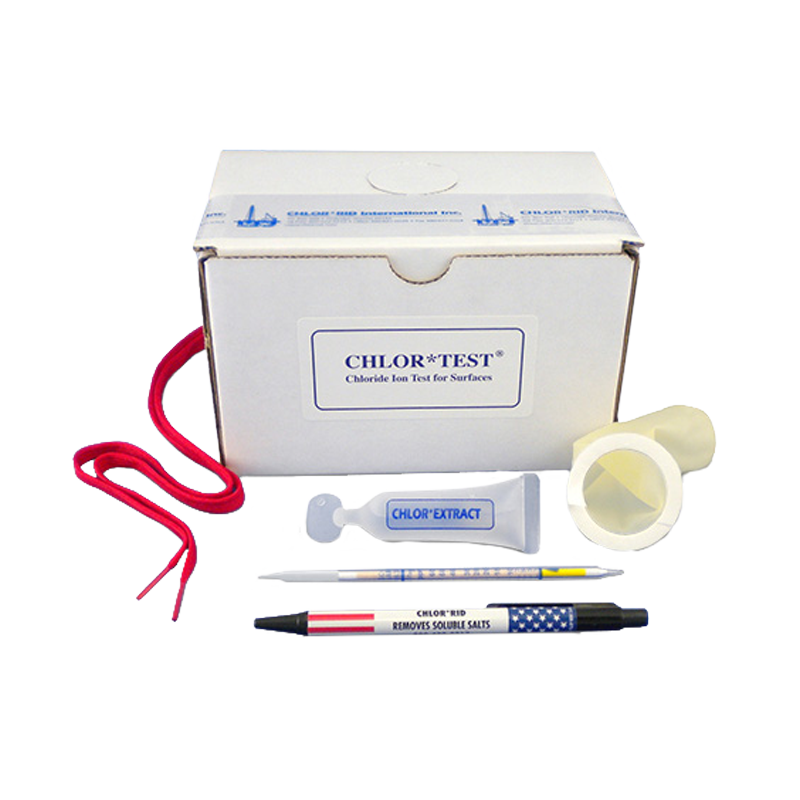
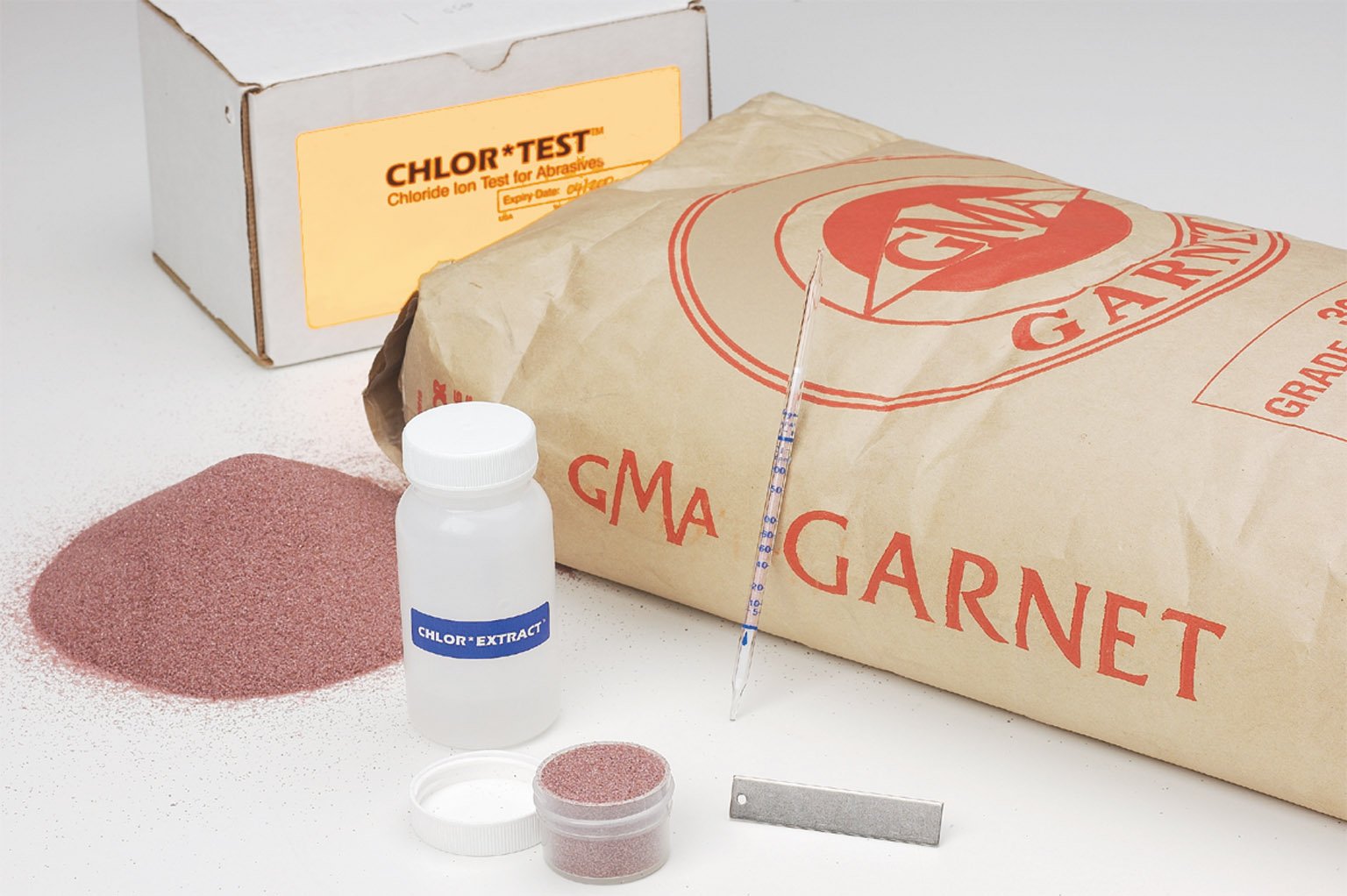
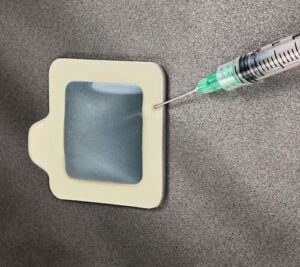
Sulfates are produced by natural sources and are generated from stack gas and diesel emissions (oxidized from sulfur dioxide) and nitrates from diesel and auto emissions (oxidized from nitrous oxide). They convert to weak sulfuric and nitric acid in the atmosphere, when in contact with moisture. They are deposited on surfaces as acid rain. Chlorine and sulfuric acid are the two most widely produced and used chemicals in the world.
Nitrogen fertilizers, in their manufacture, transport, use and runoff, result in oxidized nitrates, another salt, which is corrosive on metallic surfaces. Even without human intervention, nitrogen is in a constant cycle, alternating between soil and atmosphere. Nitrogen is constantly available in the atmosphere for deposition, and lightning readily converts atmospheric nitrogen to nitrogen oxides,which can form nitric acid. These salts act in the same way as sodium chloride (common salt) to cause corrosion. While not as prevalent a problem chloride contamination, they can be a real problem in specific environments e.g. fertilizer plant.
 My Account
My Account